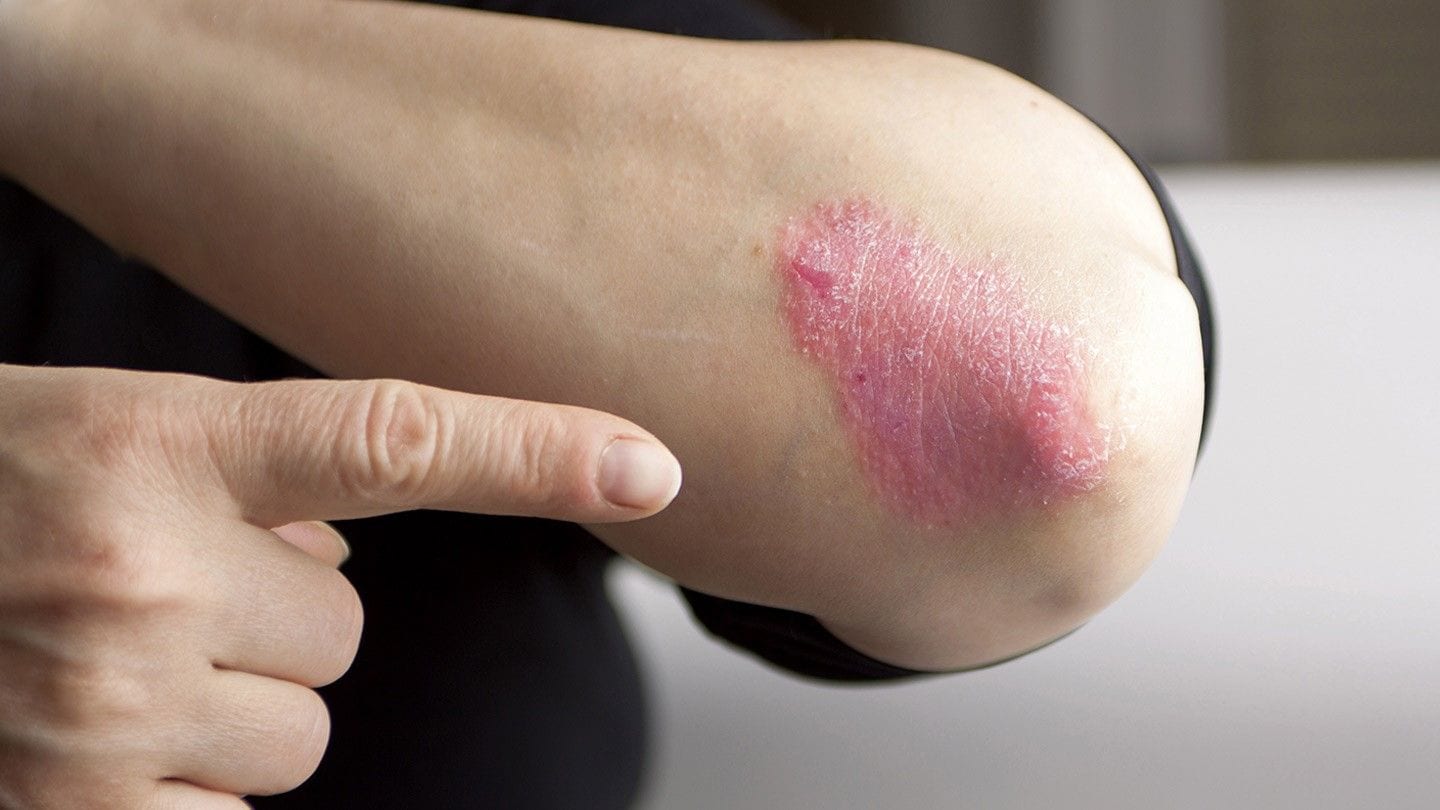
- Psoriasis appears as red and scaly patches on the skin(1). Corticosteroids and phototherapy may help relieve the symptoms of psoriasis(2).
- CBD interacts with the ECS to help manage pain, itchiness, and inflammation. These beneficial effects of CBD may help with psoriatic disease(3).
- However, more data and controlled clinical studies are needed to conclude that CBD oil may help alleviate the symptoms associated with psoriasis.
- Individuals considering trying CBD for psoriasis should first consult with their doctor for guidance and advice.
What Is CBD?
Cannabidiol or CBD is one of the commonly-known compounds of the cannabis plant. Studies suggest that the therapeutic effects of CBD may include anti-pain, anti-anxiety, anti-seizure, and anti-inflammatory properties(4).
CBD is a nonintoxicating compound, unlike tetrahydrocannabinol (THC) that contains psychoactive effects.
According to an article by the National Psoriasis Foundation, psoriasis is a systemic disease that can be linked to several comorbidities. Thus, cannabis-derived products should be used as an adjunct or complementary approach to treatment(5).
The World Health Organization recognizes CBD as an effective treatment for epilepsy. Preliminary data suggests that CBD may help with other medical conditions(6).
How CBD Oil Helps to Alleviate Symptoms of Psoriasis
CBD binds to the cannabinoid receptors of the endocannabinoid system (ECS) to help manage pain, itchiness, and inflammation(7).
The ECS acts in the immune and nervous systems and is found in the brain, organs, glands, connective tissues, and immune cells(8).
Psoriasis is an inflammatory skin disease with symptoms associated with itchiness and pain.
Studies suggest CBD may hold promise for skin-related diseases, such as psoriatic disease(9).
CBD Oil for Psoriasis: What Research Says
A 2019 study examined CBD’s effects on chronic skin diseases and cutaneous scars. The study focused mainly on psoriasis and atopic dermatitis (eczema)(10).
The study showed that topical application of CBD without THC may be a safe and effective alternative to improving the quality of life of individuals with inflammatory skin diseases.
Another study was conducted on the effectiveness of shampoo with broad-spectrum CBD. Results suggest CBD may help manage symptoms of scalp inflammation caused by seborrheic dermatitis or moderate scalp psoriasis(11).
Seborrheic dermatitis is a condition characterized by scaly, red patches on the scalp.
The researchers suggested that replacing current shampoo with broad-spectrum CBD shampoo may help reduce the severity of symptoms associated with scalp inflammation in two weeks.
In another study, researchers noted that CBD and cannabinol (CBN) may help suppress human keratinocyte proliferation(12).
Keratinocytes are the skin cells in the epidermis or outermost skin layer. According to research, the excessive growth or reproduction of keratinocytes can contribute to inflammation(13).
Several studies suggested CBD may help reduce anxiety and depression associated with various dermatologic diseases, including atopic dermatitis and psoriasis(14).
The researchers also acknowledged that evidence on the effectiveness of CBD on psoriasis-induced anxiety is still lacking.
Safety and Effectiveness
Based on a 2019 study, the individuals who administered topical CBD-enriched ointment on severe skin chronic diseases and scars twice daily for three months showed no allergic or irritant reactions within the test period(15).
A 2020 study on the effectiveness of broad-spectrum CBD shampoo showed that the product may provide excellent tolerability and satisfaction among humans(16).
The World Health Organization (WHO) also acknowledges that the use of pure CBD poses no public health issues, such as comorbidities or driving under the influence(17).
What to Consider in Choosing the Best CBD Oil for Psoriasis
CBD oil, sometimes labeled as hemp oil, is usually extracted from the cannabis plant using ethanol, carbon dioxide (CO2), or hydrocarbon extraction method.
Upon extraction, CBD oil may be classified as broad-spectrum, full-spectrum, or isolate.
Broad-spectrum CBD oil usually has all the cannabinoids found in a full-spectrum CBD product except for THC. Individuals who prefer to take CBD without the psychoactive effects of THC may consider broad-spectrum CBD as an alternative.
Full-spectrum CBD oil usually contains all the compounds from the hemp plant, including THC, terpenes, and flavonoids. Terpenes are aromatic compounds, and flavonoids are compounds containing antioxidant properties.
Combining these compounds create an “entourage effect,” wherein the purported health benefits of cannabis are improved further to promote wellness(18).
Meanwhile, isolates contain pure CBD, with no other compounds added to the product.
Reputable CBD brands selling quality products must provide certificates of analysis (COAs) on their website.
COAs are third-party lab testing results that analyze the actual content of the product, including contaminants or pesticides if any.
Some brands may also offer gluten-free or non-GMO CBD products to suit their customers’ preferences.
Individuals and psoriasis patients interested in CBD’s therapeutic potential should ask their physician or dermatologist for advice before taking CBD to help with psoriasis.
How to Use CBD Oil for Psoriasis
There are several types of high-quality CBD products that may help alleviate the symptoms of psoriasis. Product types include CBD sprays, tinctures, capsules, edibles, topicals, and vapes.
Use a dropper or oromucosal spray to administer CBD oil or CBD tinctures sublingually (under the tongue). Sublingual administration allows CBD oil to bypass the digestive tract and directly enter the bloodstream.
CBD oil can be mixed with a carrier oil such as hempseed oil or coconut oil to dilute the CBD content.
Hempseed oil extracted from hemp seeds usually does not contain CBD. However, it may provide some health benefits due to the omega-3 and omega-6 fatty acids it contains(19).
CBD capsules or edibles are taken orally. The liver absorbs them after passing through the digestive tract. Some edibles include gummies, candies, and chews.
Topicals are applied to the skin. This method is usually applicable for joint pains and skin issues. CBD applied topically is usually not absorbed into the bloodstream. Examples include CBD creams, ointments, lotions, and balms.
Vaping administers CBD oil into the body through inhalation. Vaping may be considered the fastest method for getting CBD into the body(20).
However, caution is advised when using vapes due to the potential health risks associated with lung disease(21). Individuals must consult a physician or dermatology expert before smoking or vaping CBD.
CBD Topical Cream vs. CBD oil for Psoriasis
CBD oil administered orally may take about 30 to 90 minutes to take effect and lasts for about six to eight hours(22). The drug passes through the stomach and gets metabolized by the liver before getting absorbed into the bloodstream.
Administered sublingually, CBD oil is absorbed directly into the bloodstream. CBD applied this way may take about 15 to 30 minutes and lasts for two to four hours(23).
Meanwhile, the topical application of CBD is ideal for targeted localized use. It takes 15 to 30 minutes for CBD to take effect and may last for two to four hours(24).
Pros and Cons of CBD for Psoriasis
The Pros
- CBD is a nonintoxicant and does not have the same psychoactive effects as THC(25).
- WHO acknowledges that using pure-CBD products poses no public health issues, including comorbidities or driving under the influence(26).
The Cons
- The FDA has not approved any CBD medication other than Epidiolex for treating epileptic seizures(27).
Additional studies on the therapeutic benefits of CBD are needed to determine its effectiveness and safety in managing psoriasis.
Legality
The Farm Bill passed in 2018 removed hemp-based CBD products from the list of controlled substances of the Drug Enforcement Administration (DEA).
However, marijuana-based CBD products and supplements containing more than 0.3% tetrahydrocannabinol (THC) are prohibited(28).
To date, Epidiolex is the only CBD-based medication for treating epileptic seizures approved by the Food and Drug Administration (FDA)(29).
The National Psoriasis Foundation notes state and federal regulations regarding CBD use can be conflicting. This situation may have led to an increasing number of skincare products not being tested for safety and efficacy(30).
Consumers are advised to review their state’s laws and check a product’s CBD and THC content to determine whether CBD is legal in their state.
Psoriasis Overview
Psoriasis is a skin disease that physically manifests as red and scaly patches that commonly develop on the knees, elbows, scalp, and trunk(31).
Trending statistics show that more than 8 million people in the United States have psoriasis(32).
The illness is characterized by the overproduction of the interleukin (IL)-6 and IL-8 cytokines from keratinocytes and the overactivation of neutrophils (white blood cells)(33).
The IL-6 and IL-8 cytokines are associated with the body’s inflammatory responses.
Symptoms of Psoriasis
Symptoms of psoriasis include(34):
- Dry and cracked skin that bleeds when scratched
- Stiff or swollen joints
- Itchiness, soreness, or a burning sensation
- Thickened or pitted nails
Individuals with psoriasis may experience the symptoms in cycles, usually going for weeks or months, before the illness goes into remission.
There is currently no cure for the treatment of psoriasis(35). However, some medications and alternative remedies may help manage the symptoms.
Types of Psoriasis
Psoriasis can be classified into different types, such as the following(36):
- Plaque psoriasis – Red skin lesions appear raised and covered with silvery scales. The plaques usually manifest on the elbows, knees, scalp, or lower back.
- Nail psoriasis – Psoriasis may cause the fingernails or toenails to pit or grow abnormally. The nails may crumble or separate from the nail bed in some cases.
- Inverse psoriasis – The red skin patches may appear on the folds of the buttocks, groin, or breasts. This type of psoriasis may worsen with friction or sweating and cause fungal infections.
- Guttate psoriasis – This type of psoriasis is usually caused by bacterial infection and mainly appears in children and young adults.
Guttate psoriasis may appear as scaly, dog-shaped lesions on the arms, legs, or trunk.
- Psoriatic arthritis – Psoriasis may also cause joints to become swollen and painful, resulting in arthritis.
If not immediately addressed, psoriatic arthritis may cause joint stiffness and permanently damage the joints.
- Erythrodermic psoriasis – Red, peeling rashes may appear all over the body that may feel burning or itchy. This psoriasis type is the least common.
Causes and Triggers of Psoriasis
Psoriasis is likely caused by a problem with the immune system. However, scientists still need to determine what triggers the immune system to malfunction.
The immune system overreaction causes the skin to regenerate faster than usual. This overproduction leads to abnormal skin cell growth or buildup, causing the skin to turn scaly and appear as red patches.
Individuals with psoriasis may go for an extended period without symptoms. However, environmental factors may trigger psoriasis flare-ups. Some of these triggers include(37):
- Skin injuries, infections, or strep throat (bacterial throat infection)
- Dry or cold weather
- Medications, particularly for malaria or high blood pressure
- Withdrawal of corticosteroids, a type of anti-inflammatory drug
Risk Factors for Psoriasis
Some risk factors increase the individuals’ chance of developing psoriasis or triggering its symptoms. Risk factors include(38):
- Stress – Elevated stress levels may adversely affect the individual’s immune system and trigger the symptoms of psoriasis.
- Smoking – Cigarette or tobacco smoking may cause the initial development of psoriasis and increase the severity of the symptoms.
- Family history – Genetics may contribute to an individual having psoriasis. If both parents have the illness, the risk increases more.
Challenges of Treating Psoriasis
Psoriasis is not contagious. However, there is no cure to date for psoriasis. Instead, available treatments today may help relieve the symptoms of psoriasis(39).
The method to treat psoriasis depends on the severity and location of the rashes and the individual’s age and overall health.
Some topical treatments for psoriasis include steroid creams, moisturizers, and medicated lotions.
Over-the-counter medications like hydrocortisone creams may help reduce itching and inflammation(40).
The healthcare provider may also recommend ultraviolet phototherapy, retinoids (vitamin A-based drugs), and immunotherapy medications that help prevent autoimmune diseases(41).
However, some treatment options may have significant side effects. For example, retinoids may cause congenital disabilities, and immunosuppressants like cyclosporine may cause high blood pressure or kidney damage(42).
Alternative Therapies for Psoriasis
The following alternative remedies may help manage the symptoms of psoriasis(43):
- Fish oil supplements – Help reduce scaling by applying the oil to the affected area with a dressing. Using fish oil for six hours every day for four weeks may improve skin conditions.
- Aloe extract creams – Help reduce inflammation, itching, scaling, and redness.
- Essential oils – Usually used for aromatherapy and may relieve anxiety and stress.
CBD vs. Other Alternative Psoriasis Remedies
CBD may help address pain, itchiness, and inflammation(44). These benefits are comparable to alternative remedies that help reduce itching and scaling among individuals with mild to moderate psoriasis(45).
Alternative remedies like CBD have not been conclusively proven effective. More studies are needed to determine CBD’s effectiveness and safety in managing psoriasis.
FAQs
-
Does CBD oil help with psoriasis?
There is currently insufficient evidence to conclude that CBD oil may help with psoriasis.
However, CBD interacts with the ECS to help manage pain, itchiness, and inflammation. These beneficial effects of CBD may help with skin-related diseases, such as psoriatic disease(46).
-
How much CBD should be taken for psoriasis?
There is currently no FDA-approved CBD dosage or usage guide for psoriasis.
However, researchers suggest that CBD doses of up to 1,500 milligrams per day may be well tolerated by humans(47).
CBD topicals are often used for targeted relief of skin problems. CBD topicals have fast onset times and may quickly relieve itching and reduce inflammation(48).
-
Can CBD be used with other medications for psoriasis?
CBD products may come with a grapefruit warning, meaning CBD may interfere with the effects of other medications(49).
Individuals planning to take CBD with their prescribed medications must inform their doctor or dermatologist first. Doctors can give professional advice and prescribe the proper medication to help with the symptoms of psoriasis.
- Psoriasis: Symptoms and causes
https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840 - Psoriasis: Diagnosis and treatment
https://www.mayoclinic.org/diseases-conditions/psoriasis/diagnosis-treatment/drc-20355845 - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - Cannabidiol (CBD)-what we know and what we don’t
https://www.health.harvard.edu/blog/cannabidiol-cbd-what-we-know-and-what-we-dont-2018082414476 - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - CANNABIDIOL (CBD)Pre-Review ReportAgenda Item 5.2
https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - Getting High on the Endocannabinoid System
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997295/ - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars
https://pubmed.ncbi.nlm.nih.gov/30993303/ - Efficacy and Tolerability of a Shampoo Containing Broad-Spectrum Cannabidiol in the Treatment of Scalp Inflammation in Patients with Mild to Moderate Scalp Psoriasis or Seborrheic Dermatitis
https://pubmed.ncbi.nlm.nih.gov/33313051/ - A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars
http://www.clinicaterapeutica.it/2019/170/2/05_PALMIERI-VADALA.pdf - Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function
https://www.pnas.org/content/113/41/E6162 - CBD in the Holistic Practice of Dermatology
https://assets.bmctoday.net/practicaldermatology/pdfs/PD0720_CF_CBD.pdf - A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars
https://pubmed.ncbi.nlm.nih.gov/30993303/ - Efficacy and Tolerability of a Shampoo Containing Broad-Spectrum Cannabidiol in the Treatment of Scalp Inflammation in Patients with Mild to Moderate Scalp Psoriasis or Seborrheic Dermatitis
https://pubmed.ncbi.nlm.nih.gov/33313051/ - CANNABIDIOL (CBD)Pre-Review ReportAgenda Item 5.2
https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf - The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7324885/ - Cannabinoid Profiling of Hemp Seed Oil by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry
https://www.frontiersin.org/articles/10.3389/fpls.2019.00120/full - Best way to take CBD
https://www.projectcbd.org/guidance/best-way-take-cbd - Can vaping damage your lungs? What we do (and don’t) know
https://www.health.harvard.edu/blog/can-vaping-damage-your-lungs-what-we-do-and-dont-know-2019090417734 - page 191 of Healing With CBD
https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view - page 192 of Healing With CBD
https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view - page 193 of Healing With CBD
https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - CANNABIDIOL (CBD)Pre-Review ReportAgenda Item 5.2
https://www.who.int/medicines/access/controlled-substances/5.2_CBD.pdf - FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)
https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd - CBD & THC: Myths and misconceptions
https://www.projectcbd.org/cbd-101/cbd-misconceptions - FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy
https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - Psoriasis: Symptoms and causes
https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840 - Psoriasis Statistics
https://www.psoriasis.org/psoriasis-statistics/ - A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars
https://pubmed.ncbi.nlm.nih.gov/30993303/ - Psoriasis: Symptoms and causes
https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840 - Ibid.
- Ibid.
- Ibid.
- Ibid.
- Ibid.
- What psoriasis treatments are available without a prescription?
https://www.aad.org/public/diseases/psoriasis/treatment/medications/non-prescription - Psoriasis
https://my.clevelandclinic.org/health/diseases/6866-psoriasis - Ibid.
- Psoriasis: Diagnosis and treatment
https://www.mayoclinic.org/diseases-conditions/psoriasis/diagnosis-treatment/drc-20355845 - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - Psoriasis: Diagnosis and treatment
https://www.mayoclinic.org/diseases-conditions/psoriasis/diagnosis-treatment/drc-20355845 - CBD for Psoriasis and PsA
https://www.psoriasis.org/advance/cbd-for-psoriasis-and-psa/ - Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent
https://doi.org/10.2174/157488611798280924 - page 210-211 of Healing With CBD
https://drive.google.com/file/d/1AGlxnhS2SoFeOXEuysv75bd_C9pEnwsU/view - Grapefruit Juice and Some Drugs Don’t Mix
https://www.fda.gov/consumers/consumer-updates/grapefruit-juice-and-some-drugs-dont-mix













